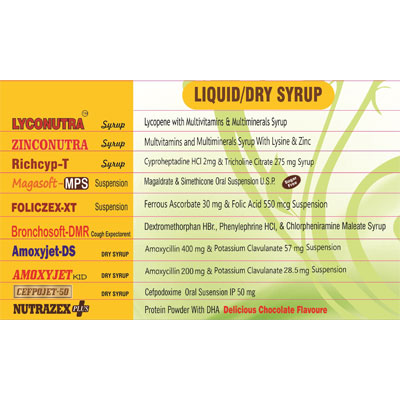
| Product Type: | Dry Syrup |
| Brand Name: | Adept Biocare |
| Packaging Type | Bottle |
| Packaging: | 15 ml |
| Composition / Material Type: | B-Complex with Lysin HCL Syrup |
| Usages: | oral use |
Amticlav
Specifications:
Amoxicillin 500 mg clavulinic acid 125 mg
FDA approved for secondary bacterial infection of acute bronchitis.
Amoxicillin with clavulinic acid,B lactamase inhibitor possesser broad spectrum activity against both gram positive gram negative organisms.
Possesses high eradication rates against multiple organisms responsible for community acquired pneumonia.
(Chronic) (Community acquired pneumonia) (sinusitis) (skin soft tissue infections ) (surgical prophyaxis)
Potent action to tread threatrning infections.
Specifications:
Amticlav 625 Tablets comes in packing 10 strips of 6 tablets each.
Clavulanic acid has a high affinity for and binds to certain β-lactamases that generally inactivate amoxicillin by hydrolyzing its β-lactam ring. Combining clavulanate potassium with amoxicillin extends the antibacterial spectrum of amoxicillin to include many bacteria normally resistant to amoxicillin and other penicillins and cephalosporins.
Distribution
Protein binding: About 25% (Clavulanic acid); about 18% (Amoxicillin). Amoxicillin distributes readily into most body tissues and fluids except the brain and spinal fluid.
Excretion
Half-life after oral admin: 1.3 hours (Amoxicillin); 1 hour (Clavulanic acid). About 50-70% of amoxicillin and 25-40% of clavulanic acid are excreted unchanged in urine during the 1st 6 hours after admin.
Precautions:
Prescribing amoxicillin and clavulanate potassium tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. While amoxicillin and clavulanate potassium tablets possess the characteristic low toxicity of the penicillin group of antibiotics, periodic assessment of organ system functions, including renal, hepatic and hematopoietic function, is advisable during prolonged therapy.
A high percentage of patients with mononucleosis who receive ampicillin develop an erythematous skin rash. Thus, ampicillin-class antibiotics should not be administered to patients with mononucleosis.
The possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur (usually involving Pseudomonas or Candida), the drug should be discontinued and/or appropriate therapy instituted.
Special Precautions:
History of allergy especially to cephalosporins, infectious mononucleosis, severe renal impairment.
Dosage:
Upper and lower respiratory tract infections
Adult: Based on amoxicillin dose, 250-500 mg every 8 hours or 500-750 mg every 12 hours.
Child: Based on amoxicillin dose: 125-250 mg every 8 hours. Children weighing <40 kg: 20-40 mg/kg/day in divided doses every 8 hours. Infants 3 months: up to 30 mg/kg/day in divided doses every 12 hours.
Storage
Store below 25°C.