| Product Type: | Injection |
| Brand Name: | Southern Pharma |
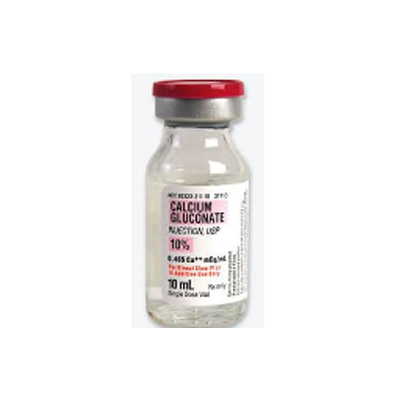
| Packaging Type | Bottle |
| Packaging: | 10 ml |
| Composition / Material Type: | Calcium Gluconate |
| Usages: | Clinical, Hospital |
Renal dialysis patient in cardiac arrest or bradycardia.
Calcium Channel Blocker OD
Hydrofluoric Acid exposure with tetany OR cardiac arrest.
Tetany may present as: overactive neurological reflexes, spasms of the hands and feet, cramps, and laryngospasm.
Prophylactically, after exposure to Hydrofluoric Acid
ADULT:
Cardiac Arrest, CCB OD and Hydrofluoric Acid exposure with tetany or cardiac arrest: 1 g (10 ml) IV
Hydrofluoric Acid Exposure Prophylaxis: 400 mg IV (4 ml)
PEDI:
Cardiac Arrest & OD: 20 mg/kg IV (max dose 500 mg in Calcium Channel Blocker OD)
THERAPEUTIC ACTION:
Antagonizes cardiac toxicity in hyperkalemia associated with dialysis patients. Reverses symptoms of Calcium Channel Blocker